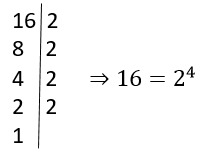- Anúncios -
O que significa off?
O termo "off" é comumente usado na linguagem coloquial brasileira, mas o que exatamente significa? Bem, o significado pode variar dependendo do contexto. Pode indicar ausência, desconexão ou até mesmo uma pausa na rotina. É uma palavra curta, mas com um significado abrangente. Vamos explorar suas diferentes nuances e descobrir como incorporá-lo em nosso vocabulário do dia-a-dia.
O que significa different?
O que significa different? Essa palavra tão simples e cotidiana guarda uma complexidade fascinante. Do latim "differentia", que significa "distinguir-se", different é um adjetivo que nos leva a pensar nas infinitas formas de singularidade que o mundo apresenta. Cada ser, objeto ou ideia carrega consigo um grau de diferença, uma peculiaridade que o torna único. Por isso, ao nos depararmos…
O que significa regular?
Você já se perguntou o que significa a palavra "regular"? Ela pode ter diferentes significados, dependendo do contexto. Pode ser algo que é comum, algo que segue uma norma ou até mesmo algo que está em bom estado. Descubra mais sobre o seu significado e exemplos de uso nesta interessante leitura!
Medicina
O que significa pancreatite aguda ou crônica?
A pancreatite é uma condição que desafia a saúde do pâncreas, um…

Create an Amazing Newspaper
Siga-nos
O que significa função afim na matemática?
A função afim na matemática é como uma dança matemática encantadora, na…
O que significa tangente na matemática?
A tangente, em sua essência matemática, é uma relação misteriosa entre duas…
O que significa limite na matemática?
Na matemática, o limite é como uma fronteira que nos permite explorar…
O que significa equações polinomiais?
Você já se perguntou sobre o significado das equações polinomiais e como…
O que significa propriedades matemáticas?
Você já se perguntou o que significa propriedades matemáticas? Neste artigo, exploraremos…
O que significa derivada na matemática?
Derivada na matemática é como uma chave mágica que nos permite desvendar…
O que significa equação?
O que significa equação? Quando mergulhamos no universo da matemática, nos deparamos…
O que significa radiciação?
A radiciação é como um delicado desvendar, um mergulho profundo em busca…

Create an Amazing Newspaper
Conteúdo patrocinado
O que significa capitalismo?
O que significa capitalismo? É um conceito que se desdobra em um verdadeiro oceano de interpretações e debates. Desde sua origem na Revolução Industrial até os dias atuais, o capitalismo abrange os pilares da propriedade privada, da livre iniciativa e do mercado competitivo. Mas, será que esse sistema econômico tem se mostrado eficiente e sustentável para todos? Exploraremos essas questões e muito mais neste artigo, mergulhando nas profundezas desse complexo sistema que molda nossa sociedade contemporânea.
O que significa PROUNI?
Você já se perguntou o que significa PROUNI? O Programa Universidade para Todos tem como objetivo oportunizar o acesso ao ensino superior para estudantes de baixa renda. Quer saber mais…
Top Autores
Stay Up to Date
Subscribe to our newsletter to get our newest articles instantly!