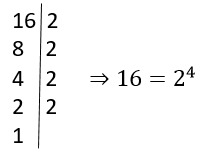- Anúncios -
O que significa glass?
Quando ouvimos falar em "glass", qual é a primeira coisa que nos vem à mente? Descubra aqui o significado por trás dessa palavra tão presente no nosso dia a dia.
O que significa camp?
Você já se perguntou o que significa camp? Esse termo pode ter diferentes significados, dependendo do contexto. Pode ser uma forma de acampar na natureza, um tipo de evento ou uma referência a um estilo de moda retrô. Seja qual for a definição, o camp sempre traz consigo uma sensação de nostalgia e aventura. Descubra mais sobre esse conceito intrigante…
O que significa acquire?
Adquirir: um verbo que significa mais do que simplesmente obter algo. É um ato de capturar, dominar e tornar parte de si. É o poder de transformar, de adicionar valor à vida. Uma palavra pequena, mas com um significado tão profundo. Descubra como a prática dessa ação pode abrir portas e impulsionar o seu sucesso.
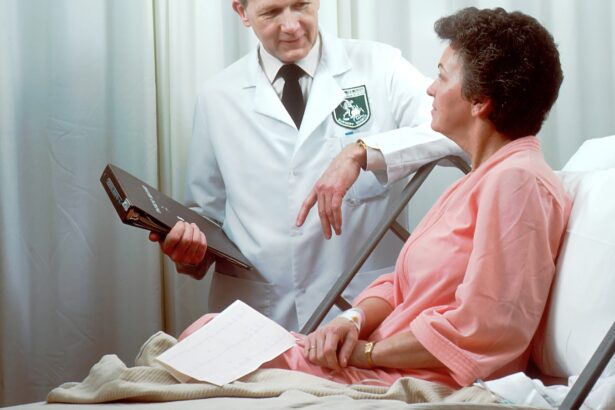
Medicina
O que significa câncer gástrico ou duodenal?
O câncer gástrico ou duodenal é uma condição séria que afeta o…

Create an Amazing Newspaper
Siga-nos
O que significa matriz na matemática?
Matriz, um conceito tão simples, mas tão profundo. Como uma teia intrincada…
O que significa acumulação na matemática?
A acumulação é um conceito matemático fascinante que nos permite entender o…
O que significa determinante na matemática?
O que significa determinante na matemática? O determinante é uma medida especial…
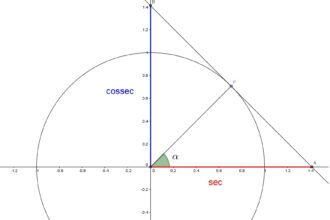
O que significa trigonometria na matemática?
O que é a trigonometria? Para alguns, é o estudo dos números…
O que significa equações exponenciais?
Você já se perguntou o que significam equações exponenciais? Essas equações misteriosas,…
O que significa vetor na matemática?
Quando entramos no mundo da matemática, nos deparamos com diversos conceitos e…
O que significa radiciação?
A radiciação é como um delicado desvendar, um mergulho profundo em busca…
O que significa axioma na matemática?
Você já se perguntou o que significa axioma na matemática? Bem, prepare-se…

Create an Amazing Newspaper
Conteúdo patrocinado
O que significa capitalismo?
O que significa capitalismo? É um conceito que se desdobra em um verdadeiro oceano de interpretações e debates. Desde sua origem na Revolução Industrial até os dias atuais, o capitalismo abrange os pilares da propriedade privada, da livre iniciativa e do mercado competitivo. Mas, será que esse sistema econômico tem se mostrado eficiente e sustentável para todos? Exploraremos essas questões e muito mais neste artigo, mergulhando nas profundezas desse complexo sistema que molda nossa sociedade contemporânea.
O que significa CST?
O que significa CST? Se você já se deparou com essa sigla e ficou intrigado, não se preocupe, você não está sozinho. CST é a abreviação para "Código de Situação…
Top Autores
Stay Up to Date
Subscribe to our newsletter to get our newest articles instantly!